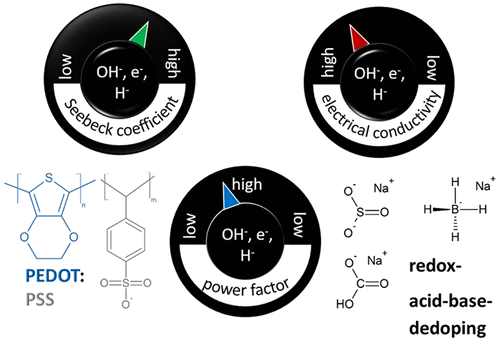
Facile optimization of thermoelectric properties in PEDOT:PSS thin films through acido-base and redox dedoping using readily available salts
Authors: Saxena, N., Keilhofer, J., Maurya, A. K., Fortunato, G., Overbeck, J., & Müller-Buschbaum, P. ;
ACS Applied Energy Materials;