Cement Hydration
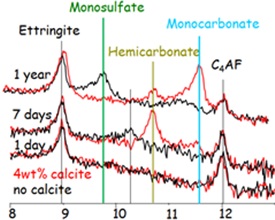
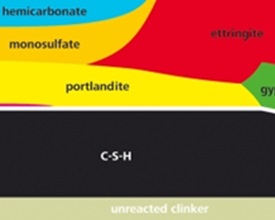
When Portland cement is brought into contact with water, its constituents start to react and various hydration products such as C-S-H (calcium silicate hydrate) gel, portlandite, ettringite, monosulfate or monocarbonate form. The composition of the cement, the interacting solution and the reaction time determine which solids (hydrates) will form, as the solid hydrates can precipitate only if the solutions are saturated or oversaturated with respect to the respective solid.
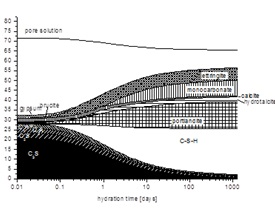
During hydration the composition of the solid and liquid phase changes. The changes in the solid phases are responsible for the development of mechanical properties. The aqueous phase of a hydrating cement can give important insights into the chemical processes and the interactions between solid and liquid phases. The composition of the liquid phase determines which hydrate phases are stable and can thus (potentially) precipitate.
The composition of hydrated cementitious systems can be complex. Thermodynamic modeling of such multicomponent-multiphase systems can promote our understanding of the impact of different factors such as composition, hydration, leaching, or temperature. In addition, adequate thermodynamic models allow easy and fast parameter variations and to extrapolate it to longer time scales, possibly reducing the amount of long and costly experimentation.
Cementitious materials are chemically reactive and have the potential to react with the environment. For example, interaction of OPC with ground, rain or soil water leads to a decrease of pH and in the long-term to the degradation of cement. First portlandite and later also C-S-H, ettringite and AFm phases are leached. The interaction with waters containing high sulfate concentrations causes the formation of ettringite, which results in expansion and cracking. In the presence of carbonate, the formation of thaumasite is also possible. Thermodynamic modeling can be used in cementitious systems to calculate the interactions with the environment.
Thermodynamic equilibrium calculations predict, based on generic data such as solubility products KS0 and complex formation constants of aqueous complexes, which complexes and solids are stable under the specific conditions of the experiment. Thermodynamic modeling is thus based on the knowledge of the thermodynamic data (e.g. solubility or complex formation) of all the solids, aqueous and gaseous species that can form in the system. These thermodynamic data are valid for all geochemical systems and compiled in different thermodynamic databases.