Power-to-fuels
While batteries are ideal for the storage of electricity from intermittent renewable sources on a time scale from seconds to days, an alternative energy storage technology is needed to balance seasonal variations in electricity production. A promising approach is to convert electric power, water, and CO2 into a synthetic fuels that can be stored in tanks for extended periods of time and ideally be transported in pipelines.
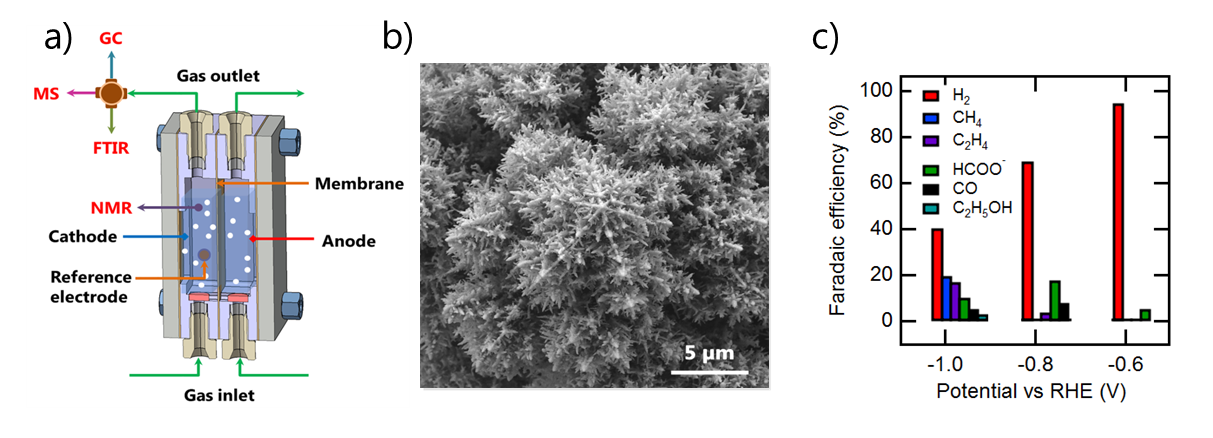
Our current research focuses on gas diffusion electrodes for the the electrochemical reduction of CO2. In previous projects, we also developed membranes for alkaline electrolysis. To study newly developed materials under realistic operating conditions, we further built a lab-scale alkaline electrolyser operating at 80 °C and 60 bar.
Funding

[1] A. Senocrate, F. Bernasconi, D. Rentsch, K. Kraft, M. Trottmann, A. Wichser, D. Bleiner, C. Battaglia, Importance of substrate pore size and wetting behavior of gas diffusion electrodes for CO2 reduction, ACS Appl. Energy Mater. 2022, in press.
[2] A. Senocrate, C. Battaglia, Electrochemical reduction fo CO2 at room temperature: status and perspectives, J. Energy Storage 2021, 36, 102373.
[3] W. Ju, F. Jiang, H. Ma, Z. Pan, Y.-B. Zhao, F. Pagani, D. Rentsch, J. Wang, C. Battaglia, Electrocatalytic reduction of gaseous CO2 to CO on Sn/Cu-nanofiber-based gas diffusion electrodes, Adv. Energy Mater. 2019, 1901514.
[4] W. Ju, J. Zeng, K. Bejtka, H. Ma, D. Rentsch, M. Castellino, A. Sacco, C. F. Pirri, C. Battaglia, Sn-decorated Cu for selective electrochemical CO2 conversion: precision architecture beyond composition design, ACS Appl. Energy Mater. 2019, 2, 867.
[5] S. Ardo, D. F. Rivas, M. A. Modestino, V. Schulze Greiving, F. Abdi, E. Alarcon-Llado, V. Artero, K. E. Ayers, C. Battaglia et al, Pathways to electrochemical solar-hydrogen technologies, Energy & Environ. Science, 2018, 11, 2768.
[6] W. Ju, M. V. F. Heinz, L. Pusterla, M. Hofer, B. Fumey, R. Castiglioni, M. Pagani, C. Battaglia, U. F. Vogt, Lab-scale alkaline electrolyzer for bridging material fundamentals with realistic operation, ACS Sust, Chem. Eng., 2018, 6, 4829.
-
Share
