Molten-salt batteries
The vulnerability of global lithium-ion battery supply chains is driving the search for alternative cell chemistries tailored to stationary energy storage. Molten-salt batteries offer a robust and sustainable solution. Manufactured using locally sourceable raw materials including nickel, iron, and rock salt, they are inherently safe, durable, and environmentally friendly. Their non-flammable solid electrolyte and anode-free assembly (forming molten sodium metal only upon first charge) enhance safety and simplify production. Operating at internal temperatures of 250–300 °C, these batteries tolerate ambient conditions from –40 °C to 60 °C, making them ideal for outdoor backup power, such as mobile telecom antennas. Despite their advantages, they face cost pressure and competition from lithium-ion batteries.
While commercial molten-salt cells rely on a complex tubular design, we developed a planar high-temperature cell platform that enables reliable operation and reproducible electrochemical cycling at laboratory scale (a). This platform allows targeted evaluation of new components under realistic conditions, from solid electrolyte processing and cathode architecture to molten sodium management and interface engineering. We combine cell cycling with detailed compositional and microstructural analysis to identify key transport and aging mechanisms (b). In collaboration with an industry partner, we contributed to the development of a cathode formulation with 20% less nickel, now implemented in commercial sodium-nickel chloride batteries, boosting energy density and cycle life while reducing material costs. A subsequent project explored fully replacing nickel with abundant zinc and further improving metal utilization at the cathode, advancing both sustainability and cost effectiveness (c).
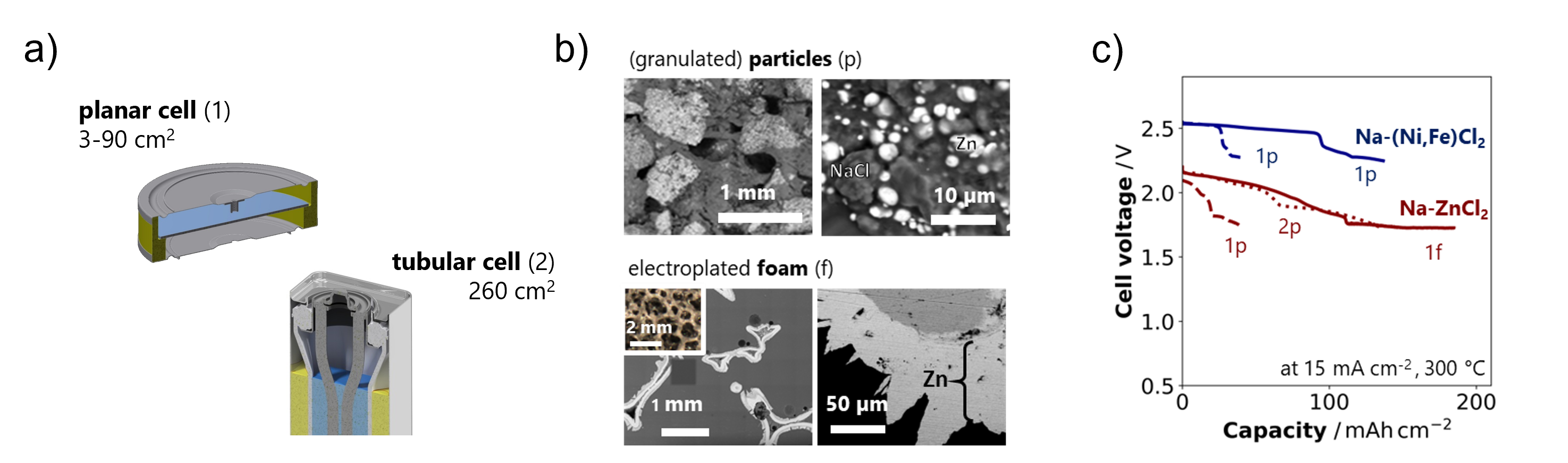
(a) Planar and tubular high-temperature molten-salt battery cell designs. (b) Cathode architecture with granulated particles and electroplated foam current collector. (c) Cell voltage vs capacity pro-files of Na-(Ni,Fe)Cl2 and Na-ZnCl2 cells with different cathode architecture, cell design, and areal capacity.
Selected publications
[1] M. V. F. Heinz, D. Landmann, D. Basso, C. Battaglia, Sodium-metal chloride (ZEBRA) batteries, in Treatise in Process Metallurgy, Elsevier, 2025, 655, https://doi.org/10.1016/B978-0-443-40294-4.00054-2
[2] S. Rahimi, E. Svaluto-Ferro, R. Celik, F. Vagliani, D. Basso, A. Turconi, A. Pozzi, C. Battaglia, M. V. F. Heinz, Enhancing specific energy and cycling stability of high-temperature Na-ZnCl2 batteries with foam-based electrodes, Advanced Energy Materials, 2025, 2501893, https://doi.org/10.1002/aenm.202501893
[3] L. Sieuw, T. Lan, E. Svaluto-Ferro, F. Vagliani, S. Kumar, W. Ding, A. Turconi, D. Basso, A. Pozzi, C. Battaglia, M. V. F. Heinz, Influence of precursor morphology and cathode processing on per-formance and cycle life of sodium-zinc chloride (Na-ZnCl2) battery cells, Energy Storage Materi-als, 2024, 64, 103077, https://doi.org/10.1016/j.ensm.2023.103077
[4] T. Lan, G. Graeber, L. Sieuw, E. Svaluto-Ferro, F. Vagliani, D. Basso, A. Turconi, C. Battaglia, M. V. F. Heinz, Planar sodium-nickel chloride batteries with high areal capacity for sustainable energy storage, Advanced Functional Materials 2023, 230240, https://doi.org/10.1002/adfm.202302040
[5] Daniel Landmann, Enea Svaluto-Ferro, Meike V. F. Heinz,* Patrik Schmutz, and Corsin Battaglia, Elucidating the rate-limiting processes in high-temperature sodium-metal chloride batteries, Ad-vanced Science 2022, 2201019, https://doi.org/10.1002/advs.202201019
[6] M. V. F. Heinz, M.-C- Bay, U. F. Vogt, C. Battaglia, Grain size effects on activation energy and conductivity: Na-β″-alumina ceramics and ion conductors with highly resistive grain boundary phases, Acta Materialia, 2021, 213, 116940, https://doi.org/10.1016/j.actamat.2021.116940
Funding
Swiss Federal Office of Energy (HiPerSonick), InnoSuisse (SoniBat), Bridge (SodiumPower), Horizon 2020 (Solstice).


Salt batteries, the fireproof battery, https://www.empa.ch/web/s604/salzbatterie
«Falling Walls 2020», Empa scientists among the winners, https://www.empa.ch/web/s604/falling-walls-2020
-
Share
