NICe- Nanointercell
The Nano-Inter-Cell (NICe) group performs cutting-edge research at the interface of particles and cellular systems for the development of innovative nanomedicines. Our ultimate goal is to provide the scientific understanding to steer the particles – cell interactions for an efficient, safe and sustainable use.
Our motivation is to establish the knowledge of how engineered (nano)particles interact with human cells and tissue to identify opportunities for health solutions and, at the same time, to avoid potential health risks in the early stage of nanomedicine's development.
Our interdisciplinary group is working together at the interface of nanomaterials, biology, toxicology, in vitro technologies, bioanalytics, nanosafety and measurement science. We address fundamental and applied questions on nanoparticles uptake, accumulation, biotransformation and their biological response, using multicellular and dynamic human in vitro models. This enables the design of novel nanomedicines, antimicrobial and antiviral materials and systems. Furthermore, we develop reliable and robust methods that enable a proper correlation of the physical-chemical properties of the nanomaterials with biological responses. The scientific knowledge gained is made accessible for the society stimulating a fact-based and transparent safety debate.
1. Safe and sustainable use of nanomaterials (Nanosafety)
1.1 Biological impact of nanomaterials
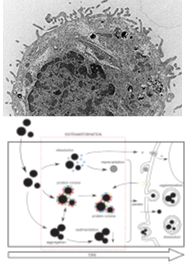
Due to their novel properties, engineered nanoparticles hold many promises for medical applications. However, exactly these novel properties raise concerns for their safe use and have led to a novel science field: nanosafety research.
We have already successfully investigated numerous projects, while currently focusing on H2020 Flagship Graphene, SafeGraph part of the EU graphene Flagship, HEurope MACRAME. The aim is to continue to investigate the correlation of specific physicochemical particle properties with their ability to evoked biological effect(s), while focusing on the understanding of protein adsorption patterns on nanoparticles, biotransformation, as well as the detection of cellular responses. In strong cooperation with our research partners we investigated in different types of nanomaterials, with well-defined properties such as metal and metal oxides, graphene related materials, carbon nanotubes and different polymer particles and we are ceaselessly motivated to keep exploring more materials.1.2 Release of nanoparticles after mechanical treatments
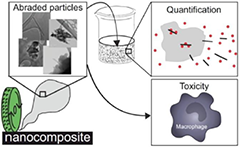
Nanomaterials are used e.g. as additives in paints, wood preservatives or to reinforce polymers. Despite the obvious benefits (e.g. obtaining a better protection of facades, reduced wood decay or improved mechanical properties of polymers) the potential hazard of these new products has to be assessed early in development.
In particular the release of nanoparticles either at the end of life or during mechanical abrasion needs to be explored in order to avoid social or economic drawbacks. Within H2020 Flagship Graphene and HEurope MACRAME we estimated the potential release of nanomaterials during mechanical treatments of nanoparticles-containing paints, antimicrobial and antiviral nanomaterials HEurope NOVA or carbon nanotubes as well as graphene reinforced polymers and assessed potential adverse effects of these processed materials. The addition of nanomaterials into paints or polymers did not lead to an additional ‘nano-risk’ due to a low amount of released particles and the absence of an acute cytotoxic response. However, the long-term consequences are still part of the ongoing research.
2. Mechanism of action of injectable nanomedicines
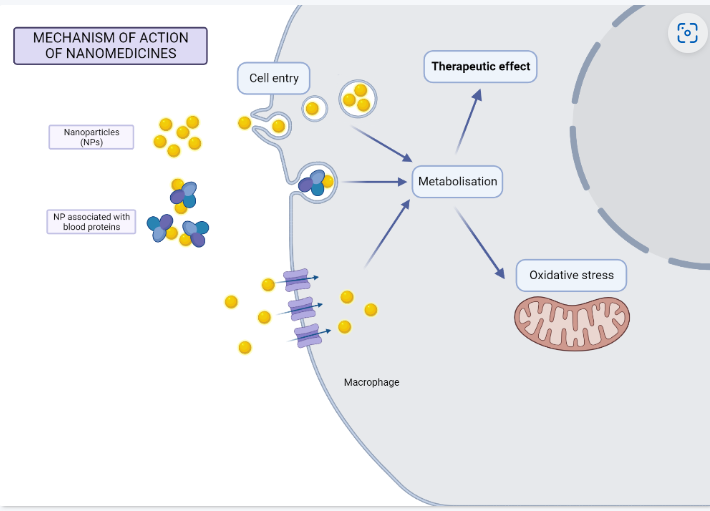
More than 100 different nanomedicines have been approved for use in patients around the world. Their safety and efficacy have been evaluated and proven in clinical trials, however, the mechanism of action of some nanomedicines at the cellular level is not fully understood. How do cells in the target organs internalize nanodrugs? How are the nanomedicines metabolized by the cells? How do they exert their therapeutic effect in the cells?
In the Nanointercell Group, we study the mechanism of action of nanotherapeutics applied in humans by using a dynamic co-culture model mimicking the blood vessel and circulating immune cells. To this end, we collaborate with pharmaceutical companies producing nanomedicines (e.g. iron nanoparticles). We are particularly interested on the response of human immune macrophages to nanomedicines, since these cells have an active role of internalizing blood-circulating nanoparticles in different organs, and are central to the clearance of nanoparticles from the organism.
In this project, we are working with state-of-the-art technical approaches like phosphoproteomic analysis, electron microscopy, flow cytometry, Raman microscopy, etc.
3. How in vitro data can be integrated into Risk Assessment: an in silico and large data approach
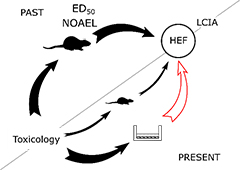
With the growing application of nanomaterials in several sectors, there is increasing attention towards the identification and management of the potential risks along the product life cycle. Life Cycle Impact Assessment (LCIA) is a methodology that evaluates the magnitude of the environmental impacts of a product/service. The impacts on human health are calculated by extrapolating human effect factors mainly from animal studies. Due to 3R and the push of alternative methods, in vivo data become scarcer, while in vitro data are increasing. However, in vitro data cannot be directly used in LCIA, and a standard integration strategy is missing. This represents a challenge especially for emerging technologies such as nanomaterials, because the lack of data results in the omission of potential impacts that, if known, could influence decision-making.
In the Horizon 2020 projects Nanorigo, HEurope MACRAME, and in collaboration with ERAM and ALCA groups, we take the first steps towards the shift of Life Cycle Impact Assessment towards an in vitro-based assessment of nanomaterial impacts on human health. Our strategy for a quantitative in vitro-in vivo extrapolation takes into account both toxicodynamics and toxicokinetics aspects of nanomaterials, by integrating in vitro toxicity data and in silico models. As inhalation is considered the main route of exposure to nanomaterials, for example in the working environment, we put a particular focus on inhaled nanoparticles and their health effects.
4. How we serve health crisis: antimicrobial and antiviral solutions
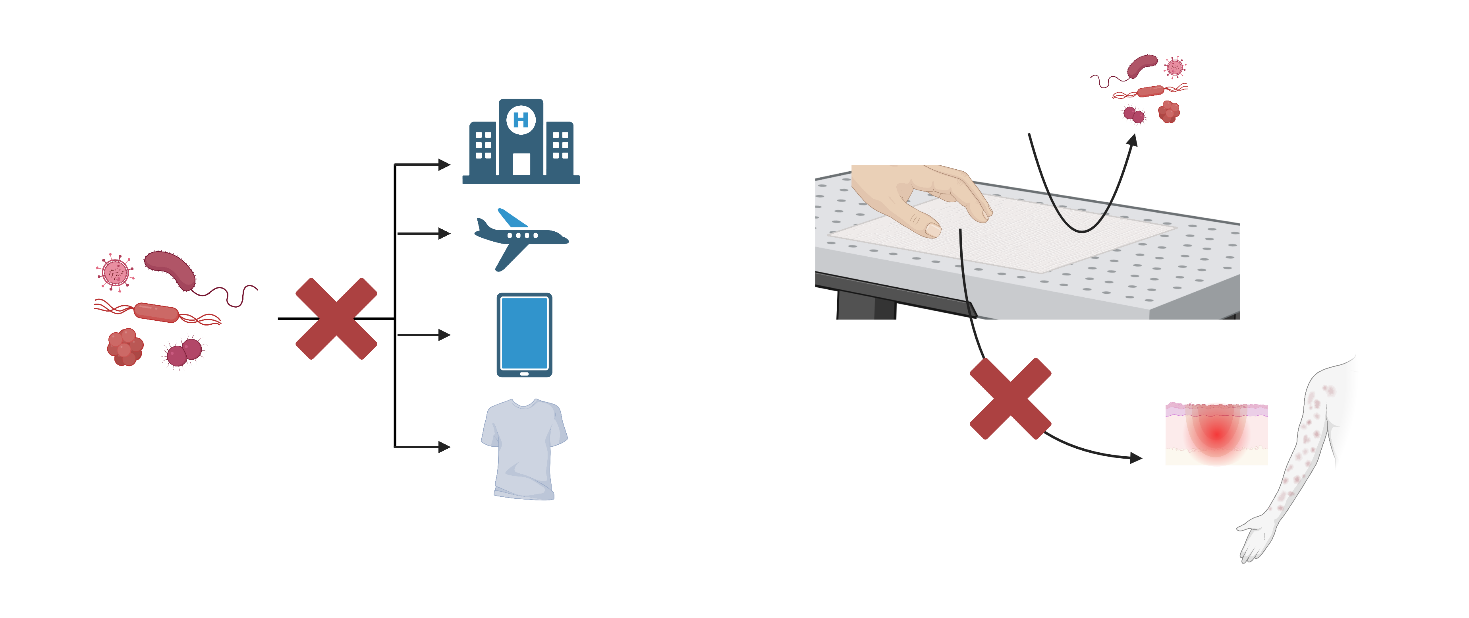
The NICe team is participating in different international projects for the development of streamlined, holistic antimicrobial coatings (antibacterial, antiviral, antifungal) for everyday settings. These projects were boosted by the COVID-19 pandemic and the subsequent identification of the unmet need to prevent microbial spreading. Such newly developed coatings will be used in everyday settings including: medical environments, public spaces (transport and buildings), on tactile surfaces (touchscreens, etc.), and on textiles (bus seats, clothes, etc.). Besides, these coatings will be in regular contact with the skin and they should not elicit adverse reactions (itching, inflammation, or contact dermatitis). Within the HEurope NOVA and RIC-2D project, we are currently studying the interaction of these coatings with different skin models, studying their irritation and sensitisation potential.
5. Human advanced in vitro models
5.1 A glioblastoma-on-chip model
Glioblastoma is a highly aggressive and malignant brain tumor that is often diagnosed at an advanced stage, making it a challenging condition to treat. Despite the standard treatment options, the prognosis for glioblastoma patients remains poor, and the disease progression is highly variable between individuals.
Personalized treatment strategies based on the characteristics of the patient's tumor can provide more accurate and effective treatment options. Aiming for a personalized and more targeted approach, the Glioblastoma project is focusing on the generation of an in vitro avatar of the patient's tumor. In collaboration with the Kantonsspital St.Gallen (KSSG), we generate patient-derived glioblastoma organoids that recapitulate the tumor's complexity and heterogeneity. The patient-derived organoids will be implemented in a vascularized tumor-on-a-chip system. The organotypic glioblastoma model system arising from this project will assist in determining patient-specific treatment modalities. This project will provide clinicians with patient-based treatment predictions, helping in the fight against this debilitating disease.
5.2 Skin, lung and GI models
The biological barriers such as lung, GI, skin or blood –tissue barrier are important structures regulating the uptake and or translocation of nanomaterials. These barriers prevent the organisms of uncontrolled exposures of any type of compounds and determine their biodistribution.
Therefore, we investigated over the years in state of the art advanced human co-culture in vitro models needed for the hazard assessment of emerging advanced materials.
6. Knowledge Transfer

We demonstrate our dedication to disseminate the latest know-how in the field of nanotechnology and safety, not just in the scientific community, but also to industry and society, by taking the lead in contactpointnano.ch.
contactpointnano.ch is a national platform, pooling the scientific and regulatory knowledge and expertise available in Switzerland on the safe handling of synthetic nanomaterials (from production to use and disposal) and conveying it efficiently and in an understandable form to companies (start-ups, SMEs, and established firms). Contactpointnano.ch accelerates the knowledge transfer and eases the way from invention to innovation, through enabling access to nano experts in Switzerland, and gives access to thematic workshops, trainings and events, concomitantly to tailored advice and support.
For further information visit the contactpointnano.ch homage.
Prof. Dr. Peter Wick, Head of Laboratory, Group Leader NICe
Alma Mater: Uni Fribourg
Keywords: Nanoparticles, Nanomaterial characterization, NanoSafety, Safe-by-Design, human in vitro models, assay reliability, nanomedicine
Dr. Nilda Vanesa Ayala Nunez, Scientist
Alma Mater: University of Groningen
Keywords: Nanosafety, in vitro endothelial models (mini-blood vessels), microfluidics
Dr. Giacomo Reina, Scientist
Alma Mater:
Keywords:
Dr. sc. Vera Maria Kissling, EM Techn. Specialist II
Alma Mater: ETH Zurich (Dr. of Sciences), University of Zurich (MSc in Biochemistry)
Keywords: Material-Biology Interactions, Biological and Material Sample Preparation, Electron Microscopy (TEM/SEM/cryo), Correlative Imaging, Cell Biology, Protein Biochemistry, Scientific/Technical Consulting and Research, EM Project Management, Leading and Training of TEM User Team, TEM laboratory and instrument responsible
Stephanie Eitner, MSc
Alma Mater:
Keywords:
Sina Ruhstaller, MSc
Alma Mater: University of Zurich
Keywords: Research Assistent; in-vitro studies
Selina Camenisch, PhD
Alma Mater:
Keywords:
Philipp Meier, PhD
Alma Mater: Zurich University of Applied Sciences
Keywords: Cell biology, Protective Safe mask safety, In-vitro assays
Su Liu, PhD
Alma Mater: Trinity College Dublin
Keywords: Organ-on-a-chip, glioblastoma
Leonard Krupnik, PhD (shared with Center for X-Ray Analytics)
Alma Mater:
Keywords:
Ziting Wang, PhD
Alma Mater: Karolinska Institute
Keywords: toxicology, nanotoxicology, in vitro study
Jimeng Wu, PhD (shared with Technology and Society Lab)
Alma Mater:
Keywords:
Alexandra Rippl, Technical Expert
Keywords: Cell culture, Flow Cytometry, Confocal Microscopy, In vitro assays
Alumni
Neda Iranpour Anaraki, shared PhD Student with lab 499 (xray facilities)
Keywords: Nanoparticles, Nano-bio Interactions, Nanoparticle Synthesis, Nanomaterials Characterization, Small Angle X-ray Scattering
Daina Romeo, PhD student
Keywords: Life Cycle Impact Assessment, nanotoxicology , inhalation, IVIVE
Pietro Clement, Scientist MSc
Alma Mater: École Polytechnique Fédérale de Lausanne (EPFL)
Keywords: Material science, antiviral characterization, fluorescence microscopy, nanoparticles, face-masks, surfaces and interfaces
Tobias Hoch, Scientist MSc
Alma Mater:
Keywords:
Dr. Chrysovalanto Louka (Irene), Postdoc
Alma Mater: Swansea University and Universite Grenoble Alps
Keywords: ALI, in vitro (co)-cultures, skin, nanotoxicology, nanomaterials, Contactpointnano.ch
Dr. Cordula Hirsch
Alma Mater: Albert-Ludwigs-Universität Freiburg
Keywords: Cell biology, Primary cell culture, Nanosafety, In vitro assays
Dr. Niusha Nikravesh, Postdoc
Alma Mater: University of Birmingham
Keywords: Cell culture, Hydrogel delivery systems, Confocal microscopy, In vitro assays
Dr. Ana Milosevic, Postdoc
Alma Mater: Uni Fribourg
Keywords: contactpointnano.ch
Liliane Diener, Technical Expert (biomedical scientist)
Keywords: Transmission Electron Microscope (TEM), biological sample preparation
Pauline Franz, MSc Student
Alma Mater: University of Constance
Dr. Juan Carlos Cassano, Postdoc
Alma Mater: University of Sydney
Keywords: Cell culture and Biology, Genotoxicity, Development of cancer diagnostic assays
Dr. Jean-Pierre Kaiser, Senior Scientist
Alma Mater: University of Zurich
Keywords: Nanotoxicology, Metallic nanoparticles, Microbiology, Cell biology, Endotoxin
Sarah May, PhD Student
Alma Mater: University of Constance
Keywords: Nanotoxicology, DNA damage, DNA repair pathways, In vitro assays
Saranya Muthusamy, MSc student
Alma Mater: TU Dresden
Keywords: Nanotechnology, Nanobiology
Dr. Nils Bohmer, Postdoc
Alma Mater: Freie Universität Berlin
Keywords: Cell culture, Nanomedicine, Endocytosis, Flow Cytometry
Biological impact of nanomaterials
Jesus S, Marques AP, Duarte A, Soares E, Costa JP, Colaco MA, Schmutz M, Som C, Borchard G, Wick P, Borges O (2020) Chitosan nanoparticles: shedding light on immunotoxicity and hemacompatibility, Front Bioeng Biotechnol 8;100
Hesler M, Aengenheister L, Ellinger B, Drexel R, Straskraba S, Jost CC, Meier F, Buechel C, Wick P, Bürki-Thurnherr T, Kohl Y, (2019) Multi-endpoint toxicological assessment of polystyrene nano- and micro- particles in different biological models in vitro, Toxicol in Vitro 61,104610
Ghaemi B, Moshiri A, Herrmann IK, Hajipour MJ, Wick P, Amani A, Sharmin Kharrazi (2019) Supramolecular insights into domino effect of Ag@ZnO-induced oxidative stress in melanoma cancer cells, ACS Applied Materials & Interfaces 11 (50), 46408-46418
Siegrist S, Cörek E, Detampel P, Sandström J, Wick P, Huwyler J, (2019) Preclinical Safety evaluation strategy for Nanomedicines, Nanotoxicology 13(1)73-99
Casalini T, Limongelli V, Schmutz M, Som C, Jordan O, Wick P, Borchard G, Perale G (2019) Molecular modeling for nanomaterials-biology interactiions: opportunities, challenges and perspectives, Front Bioeng Biotechnol 7,268
Jesus S, Schmutz M, Som C, Borchard G, Wick P, Borges O, (2019) Hazard assessment of polymeric nanobiomaterials for drug delivery: what can we learn from literature so far, Front Bioeng Biotechnol 7,261
Roman DL, Roman M, Som C, Schmutz M, Hernandez E, Wick P, Casaline T, Perale G, Ostafe V, Isvoran A, Computational assessment of the pharmacological profiles of degradation products of chitosan (2019) Front BioengBiotechnol 7,214
In vitro based LCIA
Romero D, Saleri B, Hischier R, Nowack B, Wick P, (2020) A potential integrated pathway for an early in vitro-based hazard assessment of nanoparticles Environ Intern 137;105505
Saleri B, Kaiser JP, Rösslein M, Hischier R, Nowack B, Wick P, (2020) Relative potency approach for using in vitro information for definition of effect factors of human toxicity in life cycle impact assessment Nanotoxicology. 14:2, 275-286
Release of nanoparticles after mechanical treatments
Netkueakul W, Korejwo D, Hammer T, Chortarea S, Rupper R, Braun O, Clamae M, Rothen-Rutishauser B, Buerki-Thurnherr T, Wang J*, Wick P*, (2020) Release of graphene-related materials from epoxy-based composites: characterization, quantification and hazard assessment in vitro (under revision NanoScale)
Civardi C, Grolimund D, Schubert M, Wick P, Schwarze FWMR, (2019) Micronized copper-treated wood: copper remobilization into spores from the copper-tolerant wood-destroying fungus Rhodonia placenta Environmental Science Nano 6(2),425-431
Reliable and robust characterization methods
Cassano JC, Rösslein M, Kaufmann R, Lüthi T, Schicht O, Wick P, Hirsch C, (2020) A novel approach to increase robustness, precision and high-throughput capacity of single cell gel electrophoresis, ALTEX 37(1);95-109
P. Franz, A. Bürkle, P. Wick, C. Hirsch (2020) Exploring flow cytometry-based micronucleus scoring for reliable nanomaterial genotoxicity assessment. Chemical Research in Toxicology 33: 2538-2549
Wick P, Franz P, Huber S, Hirsch C, (2020) Innovative Techniques and Strategies for a Reliable High-Throughput Genotoxicity Assessment, Chem. Res. Toxicol. 33, 2, 283-285
Petersen EJ, Hirsch C, Elliot JT, Wick P, Krug HF, Aengenheister L, May S, Rösslein M, et al., (2020) Cause–and-effect analysis as a tool to improve the reproducibility of Nanobioassays. Four case studies, Chem. Res. Toxicol. 33, 5, 1039-1054
Prina-Mello A, Schmid R, Wick P, Caputo F, Boisseau P, et al (2019) On the issue of transparency and reproducibility in nanomedicine, Nat Nanotech 14(7) 629-631
C. Hirsch and S. Schildknecht (2019) In vitro research reproducibility: keeping up high standards. Frontiers in Pharmacology, 10, 1484

