Crystalline Allrounder
A brief history of perovskites
The word “perovskite” describes a naturally occurring mineral, but also a whole range of highly specialized synthetic compounds that have promising applications in electronics and photovoltaics. But what do they actually have in common? Who discovered them? And how are Empa researchers using them to create innovative technology? This is the focus of the new issue of Empa Quarterly.
In 1839, the German mineralogist Gustav Rose received a peculiar rock sample from the Ural Mountains. Embedded in the stone was a cubic crystal about seven millimeters in size made of a previously unknown mineral. Rose named the newly discovered mineral perovskite after the Russian nobleman, mineralogist and sponsor of Rose's work – Lev Perovski.
First synthesis

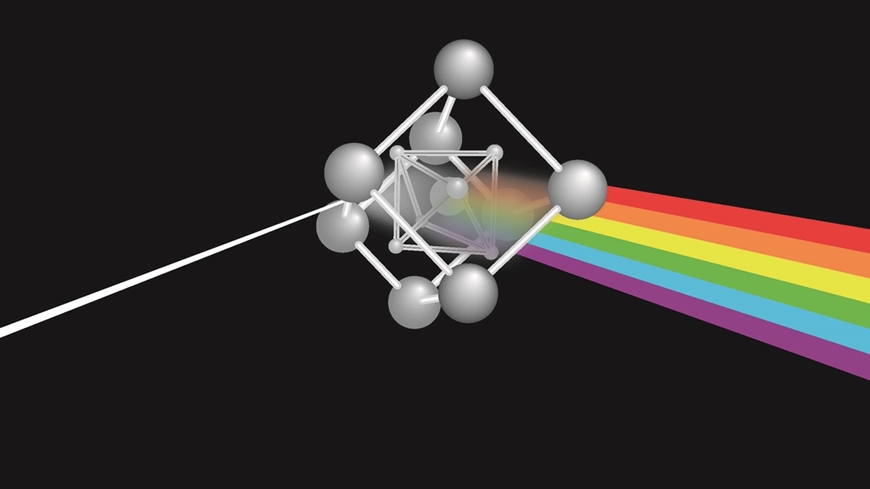
The remarkable crystal structure of perovskite was described in 1926 by the Swiss-Norwegian scientist Victor Goldschmidt. It is based on the chemical formula ABX3, where A and B are positively charged ions, also known as cations. X is a negatively charged anion. In the original perovskite, calcium titanate, A and B are calcium and titanium cations, respectively, while X is an oxygen anion.
It is also possible to synthesize perovskites from other A, B and X components. Particularly renowned are lead halide perovskites, which contain lead at the B site and X is a halogen anion such as chloride, bromide or iodide. The A site contains a large cation, usually either cesium or an organic cation such as methylammonium or formamidinium. Lead halide perovskites are good semiconductors whose properties can be fine-tuned by varying their exact composition. They are easy to produce as, for instance, thin films and large single crystals, from ordinary chemicals and solvents, or from melts.
Empa researchers who are working on various applications for lead halide perovskites are taking advantage of their unique properties. In 2014, the team of Maksym Kovalenko at ETH Zurich and Empa, including Maryna Bodnarchuk at Empa's Laboratory for Thin Films and Photovoltaics, for the first time synthesized tiny and monodisperse perovskite nanocrystals, known as quantum dots. They continue intense materials design work in this direction. Kovalenko's group is also conducting research into image sensors with thin-film perovskites as well as gamma and X-ray detectors using single-crystal perovskite layers.
Empa researcher Fan Fu and his team, on the other hand, are focusing on perovskite solar cells, which promise high efficiency and flexibility. The Laboratory for Functional Polymers, headed by Frank Nüesch, is also working on perovskite solar cells. In 2020, it gave rise to the spin-off Perovskia Solar, only around 30 years after perovskite solar cells were first described – and 181 years after Gustav Rose first obtained the unusual crystal.
Structure and applications of perovskites

Empa Quarterly#83 Perovskites: Versatile cristals
Over 180 years ago, a curious crystal was discovered in the Ural Mountains. Today, it has given rise to an entire class of materials that is of great interest to researchers: perovskites. What all perovskites have in common is their crystal structure, which gives them unusual properties. By changing the exact composition of the perovskite, scientists can control these properties. Empa researchers are using this promising material to develop solar cells, detectors and quantum dots.
Read the EmpaQuarterly online or download the pdf-version.
-
Share






