FTIR method development for industrial process optimization
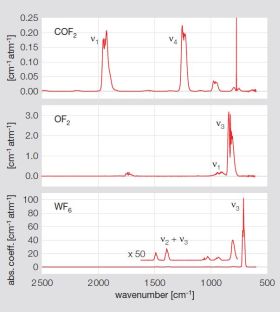
A very frequent process is the chemical vapor deposition (CVD) of tungsten. Our studies in several semiconductor fabs indicate significant optimization potential with respect to gas consumption and process time. Furthermore, unexpected reaction by-products can be identified, which in some applications were shown to be a source for decreased yield.
In waste incineration plants, nitrogen oxides (NOx) are often removed in a selective catalytic reduction (SCR) process. SCR relies on the addition of ammonia (NH3) as a reductant for nitrogen oxide (NO). DeNOx by SCR is often limited by a non-homogeneous distribution of NH3 in the catalyst. This has two highly unwanted effects: (i) it leads to inefficient reduction of NO to N2, and thus to high NOx emissions, sometimes even above the legal thresholds, and (ii) it increases the consumption of NH3, produces unnecessary NH3 slip and high costs. The distribution of NH3 in a catalyst can be determined by FTIR grid measurements, which is subsequently also used to confirm the optimization of the NH3 addition.
References:
- Mohn, J., Forss, A.M., Bruhlmann, S., Zeyer, K., Luscher, R., Emmenegger, L., Novak, P., Heeb, N., 2004. Time-resolved ammonia measurement in vehicle exhaust. Int. J. Environ. Pollut. 22 (3), 342-356.
- Mohn, J., Beck, U., Zeyer, K., Emmenegger, L., 2005. Calibration of reactive process gases for the characterization of semiconductor processes by FTIR. J. Mol. Struct. 744-747 (SPEC. ISS.), 247-253.
- Mohn, J., Galli, R., Emmenegger, L., 2006. Real-time measurement of reactive process gases in microelectronics by means of FTIR spectroscopy. Chem. Ing. Tech. 78 (10), 1524-1530
-
Share