CELL-/TISSUE MATERIAL INTERACTIONS
Selected publications
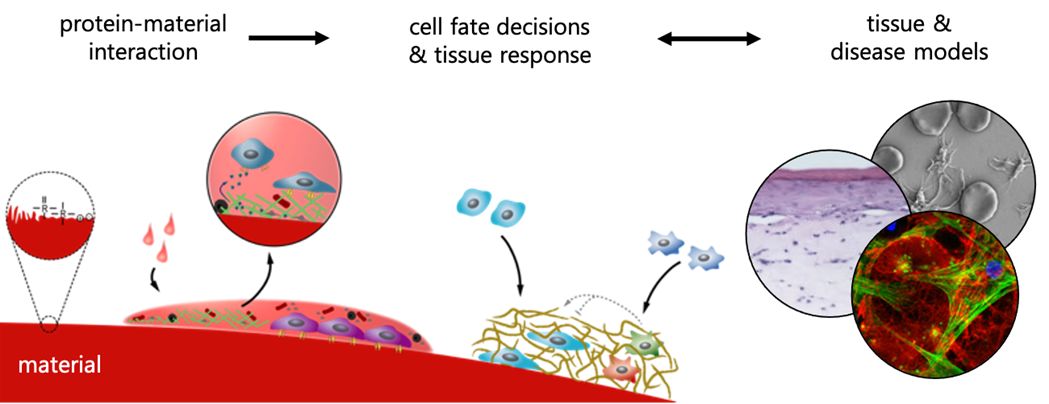
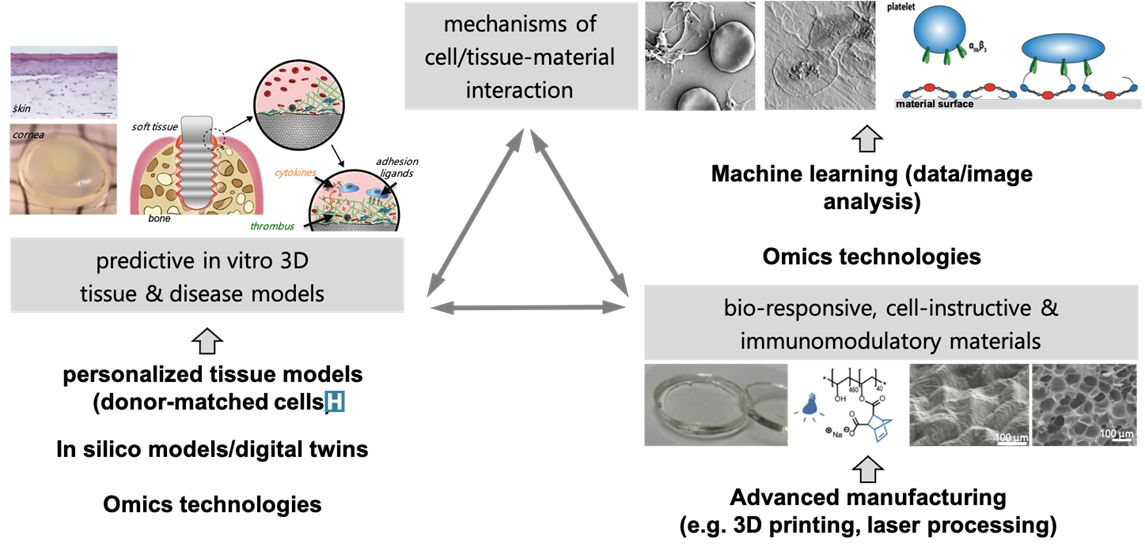
Our group aims to understand the mechanisms of how human cells and tissues interact with materials/material surfaces and, in close collaboration with clinics and industry, to use this knowledge to develop novel materials for unmet clinical needs.
We develop tissue-specific advanced in vitro models that more closely mimic the in vivo situation to investigate the interactions of cells and tissues with materials. We study the early events of blood-material interaction and its influence on human cell response governing integration or non-integration of materials into host tissue as well as cellular processes involved in wound healing, mainly focusing on bone, skin and soft tissue.
Scrutinizing cellular signaling pathways, we explore how the tissue response can be steered with surface functionalization or controlled release of bioactive molecules from materials. Ultimately, we evaluate the predictive power of our models via correlation with in vivo results and clinical data in collaboration with our academic, industrial and clinical partners. For this, we use state of the art techniques including gene- and protein expression analysis as well as microscopy techniques.
Materials to improve wound healing
When damage to the body occurs, healing processes often lead to fibrosis or tissue scarring, where normal tissue is replaced by permanent scar/fibrotic tissue with impaired functionality.
Our goal is to understand the occurrence of and mechanisms involved in impaired wound healing and tissue scarring for the development of novel, material-based treatment strategies. We explore and develop new materials and fabrication concepts to create tailor-made solutions for different wound pathologies. Furthermore, we develop new advanced in vitro 3D co-culture models and use analytical tools including omics-technology, ELISA, SEM or 2-photon microscopy.

Selected research topics:
- Controlling pH by electronic ion pumps to fight fibrosis
- Near-infrared light-sensitive polyvinyl alcohol hydrogel photoresist for spatiotemporal control of cell-instructive 3D microenvironments
- Complex 3D skin models based on a light-sensitive polyvinyl alcohol hydrogel (Skintegrity)
- 3D scaffold based on biodegradable P4HB for the treatment of chronic skin wounds and scar tissue formation (ScarAvoid)
Steering integration and non-integration of materials
Implantable biomaterials are designed to function either in a transient or permanent manner. Depending on the clinical indication, integration of an implant material into the host tissue is desired or needs to be avoided. Generally, fast and specific protein adsorption and enhanced cell migration towards the implant is beneficial in the former situation, whereas non-fouling is favored in the latter one. In both cases however, implant surface properties including roughness, surface chemistry or wettability influence the tissue response to the material. Addition of drug-release functionality to a material can be used to further enhance such a response or to add novel properties such as an antibacterial effect to the material.
Our aim is to understand how such material properties influence different stages of the tissue response to implants, ranging from blood-material interaction to immune cell recruitment to cell differentiation processes, and to develop novel materials and material surfaces to either achieve or to avoid tissue integration. For this, we develop in vitro 3D models that mimic the target tissue and that allow to recreate the situation during implantation of a material in vitro.
Selected research:
- Rationally designed ultra-short pulsed laser patterning of zirconia-based ceramics tailored for the bone-implant interface
- Surface modification of ultrafine‐grained titanium: Influence on mechanical properties, cytocompatibility, and osseointegration potential
- In Vitro Cytocompatibility Assessment of Ti-Modified, Silicon-oxycarbide-Based, Polymer-Derived, Ceramic-Implantable Electrodes under Pacing Conditions
- Enhanced endothelialization of electrospun 3D scaffold interlayers on hyperelastic membranes in pulsatile ventricular assist devices by surface functionalization or hybrid scaffold design (Zurich Heart)
Controlling the immune response towards materials
The immune response is a key element of wound healing and when the body interacts with implanted materials. Encompassing a complex and coordinated series of events, any disturbance can lead to a variety of pathologies or the development of fibrotic tissue in response to implantation of materials.
Our aim is thus to better understand how we can control the immune cell response (e.g. the switch during macrophage polarization into a pro- or anti-inflammatory phenotype) in wound healing but also in contact with engineered materials, and develop novel biomaterials with local immunomodulation capabilities (e.g. 3D scaffolds with controlled drug release).
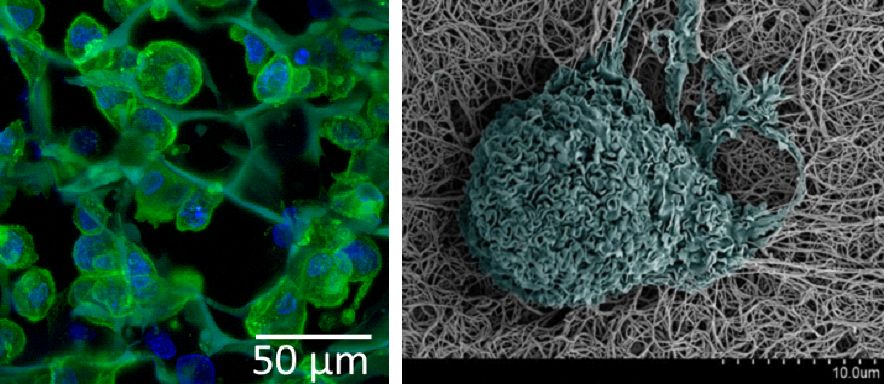
Selected research:
- In vitro skin culture media influence the viability and inflammatory response of primary macrophages
- Tissue inhibitor of metalloproteinase (TIMP) peptidomimetic as an adjunctive therapy for infectious keratitis
- Lumican is upregulated in osteoarthritis and contributes to TLR4-induced pro-inflammatory activation of cartilage degradation and macrophage polarization
- Silk based scaffolds with immunomodulatory capacity: anti-inflammatory effects of nicotinic acid
Within our different research projects, we collaborate with excellent partners from academia (e.g. Prof. Marcy Zenobi-Wong (ETH Zurich), Prof. Viola Vogel (ETH Zurich), Prof. Jörg Löffler (ETH Zurich), Prof. Jürgen Brugger (EPFL), Prof. Brigitte von Rechenberg (University Zurich), Prof. Simon Pot (University Zurich), Prof. Rainer Riedl (ZHAW Wädenswil), Prof. Heike Walles (University of Magdeburg), Prof. Sally McArthur (Swinburne University), from clinics (e.g. Dr. Karl Grob, (Cantonal Hospital St.Gallen), Dr. Peter Wahl (Cantonal Hospital Winterthur), Dr. Elisabeth Roider (University Hospital Basel), and from industry (e.g. Institut Straumann AG, Lumendo AG, Meddrop AG).
We kindly thank for the financial support by Gebert Rüf Stiftung, Helmut Horten Foundation, Novartis FreeNovation, Novartis Foundation for Medical-Biological Research, SFA Additive Manufacturing, OrthoTrauma Foundation, International Team for Implantology, SNF and Innosuisse.
Dr. Max Urbanczyk
PostDoc
Dr. Rahul Rimal
PostDoc
Marine De Lapeyrière
PhD student
Hien Phuong Le
PhD student
Stefanie Guimond
Technical Expert
Yvonne Elbs-Glatz
Technician
Leonor Veloso
Master student
Agnès Stürner
Master student
agnes.stuerner@empa.ch
Associated
Alumni
Dr. Arie Bruinink, Scientist
Dr. Géraldine Guex, Scientist
Dr. Vera Malheiro, PostDoc/Scientist
Dr. Samantha Chan, PostDoc/Scientist
Dr. Xiao-Hua Qin, PostDoc/Scientist
Dr. Eike Müller, PostDoc/Scientist
Dr. Berna Neidhart, PostDoc/Scientist
Rebecca Huber, PhD candidate
Gökce Yazgan, PhD candidate
Lukas Weidenbacher, PhD candidate
Chiara Griffoni, PhD candidate
Anne-Sophie Mertgen, PhD candidate
Amin Zakeri Ziavashani, guest PhD candidate
Marielle Hintereder, Master student
Andrej Eigenmann, Bachelor student
Tanja Schwalm, pre-study internship
Leonie Bannwarth, pre-study internship
Pascal Boucq, Master student
Moritz Valeske, Master student
Barbora Kolrosová, Master student
Lada Fleyshman, Master student
Judith Ng, PhD student
Milo Rechsteiner, Pre-study internship
Romana Arnold, Master student
Romy Wiestner, Master student
Tanja Schwalm, Master student
David Ilbrink, Master student
Celina Spangenberger, Master student
Annina Mittelholzer, Master student
Ke Yang, PhD student
Dr. med et Dr. med. dent. Matthias G. Wiesli, PhD student
Dr. William Lackington, Scientist
Jeshurun Manoranjan, Master student
Dr. Yashoda Chandorkar, Scientist
Xena Nadine Grob-Nettleton, Intern
Annina Mittelholzer, Scientific collaborator
Emina Bejtic, Master student
Jose Antonio Simon Greminger, Master student

CELL-/TISSUE MATERIAL INTERACTIONS
Selected publications
Our group aims to understand the mechanisms of how human cells and tissues interact with materials/material surfaces and, in close collaboration with clinics and industry, to use this knowledge to develop novel materials for unmet clinical needs.
We develop tissue-specific advanced in vitro models that more closely mimic the in vivo situation to investigate the interactions of cells and tissues with materials. We study the early events of blood-material interaction and its influence on human cell response governing integration or non-integration of materials into host tissue as well as cellular processes involved in wound healing, mainly focusing on bone, skin and soft tissue.
Scrutinizing cellular signaling pathways, we explore how the tissue response can be steered with surface functionalization or controlled release of bioactive molecules from materials. Ultimately, we evaluate the predictive power of our models via correlation with in vivo results and clinical data in collaboration with our academic, industrial and clinical partners. For this, we use state of the art techniques including gene- and protein expression analysis as well as microscopy techniques.


Materials to improve wound healing
When damage to the body occurs, healing processes often lead to fibrosis or tissue scarring, where normal tissue is replaced by permanent scar/fibrotic tissue with impaired functionality.
Our goal is to understand the occurrence of and mechanisms involved in impaired wound healing and tissue scarring for the development of novel, material-based treatment strategies. We explore and develop new materials and fabrication concepts to create tailor-made solutions for different wound pathologies. Furthermore, we develop new advanced in vitro 3D co-culture models and use analytical tools including omics-technology, ELISA, SEM or 2-photon microscopy.

Selected research topics:
- Controlling pH by electronic ion pumps to fight fibrosis
- Near-infrared light-sensitive polyvinyl alcohol hydrogel photoresist for spatiotemporal control of cell-instructive 3D microenvironments
- Complex 3D skin models based on a light-sensitive polyvinyl alcohol hydrogel (Skintegrity)
- 3D scaffold based on biodegradable P4HB for the treatment of chronic skin wounds and scar tissue formation (ScarAvoid)
Steering integration and non-integration of materials
Implantable biomaterials are designed to function either in a transient or permanent manner. Depending on the clinical indication, integration of an implant material into the host tissue is desired or needs to be avoided. Generally, fast and specific protein adsorption and enhanced cell migration towards the implant is beneficial in the former situation, whereas non-fouling is favored in the latter one. In both cases however, implant surface properties including roughness, surface chemistry or wettability influence the tissue response to the material. Addition of drug-release functionality to a material can be used to further enhance such a response or to add novel properties such as an antibacterial effect to the material.
Our aim is to understand how such material properties influence different stages of the tissue response to implants, ranging from blood-material interaction to immune cell recruitment to cell differentiation processes, and to develop novel materials and material surfaces to either achieve or to avoid tissue integration. For this, we develop in vitro 3D models that mimic the target tissue and that allow to recreate the situation during implantation of a material in vitro.

Selected research:
- Rationally designed ultra-short pulsed laser patterning of zirconia-based ceramics tailored for the bone-implant interface
- Surface modification of ultrafine‐grained titanium: Influence on mechanical properties, cytocompatibility, and osseointegration potential
- In Vitro Cytocompatibility Assessment of Ti-Modified, Silicon-oxycarbide-Based, Polymer-Derived, Ceramic-Implantable Electrodes under Pacing Conditions
- Enhanced endothelialization of electrospun 3D scaffold interlayers on hyperelastic membranes in pulsatile ventricular assist devices by surface functionalization or hybrid scaffold design (Zurich Heart)
Controlling the immune response towards materials
The immune response is a key element of wound healing and when the body interacts with implanted materials. Encompassing a complex and coordinated series of events, any disturbance can lead to a variety of pathologies or the development of fibrotic tissue in response to implantation of materials.
Our aim is thus to better understand how we can control the immune cell response (e.g. the switch during macrophage polarization into a pro- or anti-inflammatory phenotype) in wound healing but also in contact with engineered materials, and develop novel biomaterials with local immunomodulation capabilities (e.g. 3D scaffolds with controlled drug release).

Selected research:
- In vitro skin culture media influence the viability and inflammatory response of primary macrophages
- Tissue inhibitor of metalloproteinase (TIMP) peptidomimetic as an adjunctive therapy for infectious keratitis
- Lumican is upregulated in osteoarthritis and contributes to TLR4-induced pro-inflammatory activation of cartilage degradation and macrophage polarization
- Silk based scaffolds with immunomodulatory capacity: anti-inflammatory effects of nicotinic acid
Within our different research projects, we collaborate with excellent partners from academia (e.g. Prof. Marcy Zenobi-Wong (ETH Zurich), Prof. Viola Vogel (ETH Zurich), Prof. Jörg Löffler (ETH Zurich), Prof. Jürgen Brugger (EPFL), Prof. Brigitte von Rechenberg (University Zurich), Prof. Simon Pot (University Zurich), Prof. Rainer Riedl (ZHAW Wädenswil), Prof. Heike Walles (University of Magdeburg), Prof. Sally McArthur (Swinburne University), from clinics (e.g. Dr. Karl Grob, (Cantonal Hospital St.Gallen), Dr. Peter Wahl (Cantonal Hospital Winterthur), Dr. Elisabeth Roider (University Hospital Basel), and from industry (e.g. Institut Straumann AG, Lumendo AG, Meddrop AG).
We kindly thank for the financial support by Gebert Rüf Stiftung, Helmut Horten Foundation, Novartis FreeNovation, Novartis Foundation for Medical-Biological Research, SFA Additive Manufacturing, OrthoTrauma Foundation, International Team for Implantology, SNF and Innosuisse.
Dr. Max Urbanczyk
PostDoc
Dr. Rahul Rimal
PostDoc
Marine De Lapeyrière
PhD student
Hien Phuong Le
PhD student
Stefanie Guimond
Technical Expert
Yvonne Elbs-Glatz
Technician
Leonor Veloso
Master student
Agnès Stürner
Master student
agnes.stuerner@empa.ch
Associated
Alumni
Dr. Arie Bruinink, Scientist
Dr. Géraldine Guex, Scientist
Dr. Vera Malheiro, PostDoc/Scientist
Dr. Samantha Chan, PostDoc/Scientist
Dr. Xiao-Hua Qin, PostDoc/Scientist
Dr. Eike Müller, PostDoc/Scientist
Dr. Berna Neidhart, PostDoc/Scientist
Rebecca Huber, PhD candidate
Gökce Yazgan, PhD candidate
Lukas Weidenbacher, PhD candidate
Chiara Griffoni, PhD candidate
Anne-Sophie Mertgen, PhD candidate
Amin Zakeri Ziavashani, guest PhD candidate
Marielle Hintereder, Master student
Andrej Eigenmann, Bachelor student
Tanja Schwalm, pre-study internship
Leonie Bannwarth, pre-study internship
Pascal Boucq, Master student
Moritz Valeske, Master student
Barbora Kolrosová, Master student
Lada Fleyshman, Master student
Judith Ng, PhD student
Milo Rechsteiner, Pre-study internship
Romana Arnold, Master student
Romy Wiestner, Master student
Tanja Schwalm, Master student
David Ilbrink, Master student
Celina Spangenberger, Master student
Annina Mittelholzer, Master student
Ke Yang, PhD student
Dr. med et Dr. med. dent. Matthias G. Wiesli, PhD student
Dr. William Lackington, Scientist
Jeshurun Manoranjan, Master student
Dr. Yashoda Chandorkar, Scientist
Xena Nadine Grob-Nettleton, Intern
Annina Mittelholzer, Scientific collaborator
Emina Bejtic, Master student
Jose Antonio Simon Greminger, Master student

Bacteria and Materials Interactions
The Bacteria & Materials Interaction Group focuses on understanding, predicting and controlling material-bacteria interactions for health-related applications. In particular, we combine interdisciplinary knowledge in the pursuit of understanding how bacteria adhere to surfaces, and how the adhesion can be controlled. Among our core strengths are the expertise ranging from microbiology to biomaterials, and the long-term experience in working with hospitals, academic partner institutions, and industry.
Our key research aspects include:
- Studying mechanisms of bacterial adhesion on surfaces, particularly bacterial mechanobiology, aiming at antibacterial and anti-adhesive surfaces
- Studying mechanisms of biofilm resistance toward antimicrobials
- Development of biosensors for detection of antimicrobial resistant bacteria
- Design of novel approaches for treatment of antimicrobial resistance
Research Activities
Bacteria-Materials interactions
Upon sensing and interacting with materials, bacteria trigger a variety of changes in gene expression, including the genes essential for cell-cell communication, motility and surface attachment. Although changes in these phenotypes have been observed, the mechanisms used by bacterial cells for sensing and responding to surfaces are not well understood. To develop novel classes of materials that inhibit or reduce bacterial adhesion or proliferation, a better understanding of these mechanisms is necessary. Our group is interested in studying the underlining mechanisms of surface sensing at the molecular level and the consequent impact on cell adhesion.
We have close collaborations with Dr. Dominik Abt (Cantonal Hospital St. Gallen), Prof. Dr. Leo Eberl (University of Zurich), Prof. Dr. Henny van der Mei (University Medical Center Groningen) and Prof. Dr. Songmei Wu (Beijing Jiaotong University). We greatly acknowledge support from Innosuisse.
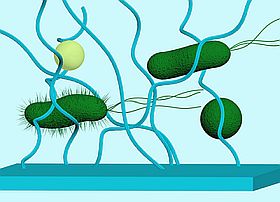
The physicochemical properties of a material surface impact bacterial adhesion, which consequent can trigger altered gene expression.
Antimicrobial resistance (AMR) of biofilm
Infections are often caused by bacterial biofilms. Bacteria living in biofilms can tolerate much higher antibiotic concentrations compared to planktonic bacteria and survive long enough to evolve antimicrobial resistance (AMR). Our group aims to investigate how bacteria generate resistant during biofilm formation on medical devices, and utilize the gained knowledge to develop novel antimicrobial strategies to prevent biofilm-associated infection and AMR.
We have close collaboration with Dr. Baharak Babouee Flury (Cantonal Hospital St. Gallen), Dr. Frank Schreiber (BAM), Prof. Dr. Henny van der Mei (University Medical Center Groningen), Prof. Jeremy Webb (University of Southampton), and Dr. Christian Ahrens (Agroscope). We greatly acknowledge the support from the Swiss National Science Foundation on our project "Partnership against biofilm-associated expression, acquisition and transmission of AMR".
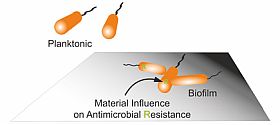
Understanding mechanism of biofilm resistance toward antimicrobial materials by identifying involved genes and pathways.
Biosensors for detection of antimicrobial resistant bacteria
We combine material science, chemistry, and biology to design non-invasive biosensors highly specific for resistant bacteria. The biosensors, including those for evaluation of skin wound infections, dental diseases, sepsis and pneumonia etiology, have potential to allow surgeons to achieve rapid diagnosis and initiate appropriate treatment timely.
Diverse projects are carried out in close collaboration with Prof. Dr. Inge Herrmann (ETHZ/Empa), Prof. Dr. Simone Schurle (ETHZ), Dr. Saso Jezernik and Dr. Christian Jäggi (Bioinitials), Dr. Silvia Generelli (CSEM), Dr. Stefan Stübinger (University of Basel), Dr. Werner Albrich and Dr. Christian Kahlert (Cantonal Hospital St. Gallen). We greatly acknowledge support from Innosuisse.
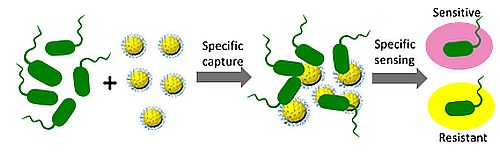
Novel approaches for treatment of antimicrobial resistance
To reduce development of antimicrobial resistance, we utilize natural materials, hazard-free and easily accessible approaches to replace antibiotics to treat biofilm associated chronic wounds. These bioinspired wound dressings not only allow elimination of biofilms but also promote tissue re-epithelization and wound healing.
This project is carried out in close collaboration with Dr. Zhihao Li (University Hospital Zürich), Prof. Jörg Grünert (Canton Hospital St. Gallen) Prof. Sven Panke and Dr. Martin Held (ETHZ). We greatly acknowledge the support from the Swiss National Science Foundation on our project "Probiotics for treatment of skin wound infection: fighting biofilms and promoting tissue re-generation" starting on June 1st, 2021.
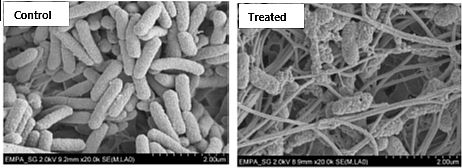
Biofilm Lab and characterization tools
Our group hosts a Biosafety Class II Lab where the group works with different bacterial strains including Gram-negative and Gram-positive species and multi-resistant pathogens. We are also equipped with a dedicated lab for co-culture of bacteria and mammalian cells. We study biofilms in microtiter plates, flow chambers, as well as microfluidic systems under static or dynamic conditions. Bacterial adhesion and biofilms on surfaces are analyzed by different methods such as Crystal violet assays, Fluorescence assays, Microscopy (Wide-field, CLSM, SEM), Real-time quantitative PCR.
Dr. Clara Guarch Perez
PostDoc
Dr. Sumanta Ghosh
PostDoc
sumanta.ghosh@empa.ch
Flavia Zuber
Laboratory technician
Microbiology, in-vitro biofilm assays, cell culture, cytotoxicity testing
Stefanie Altenried
Laboratory technician
In vitro biofilm formation and assessment, Microbiology, Biotechnology, molecule biology
Sixuan Zhang
Liubov Shishaeva
PhD candidate
Siyuan Tao
PhD candidate
Anna Hu
PhD candidate

Tissue-Regenerative Soft Materials
Selected publications
The "Tissue-Regenerative Soft Materials" Group is a joint group of the Laboratories for 'Biomimetic Membranes and Textiles' and 'Biointerfaces' in Empa's Research Focus Area 'Health'. With an interdisciplinary team of polymer scientists, biologists, and biomedical engineers, we focus on the development of functional polymers and multi-scale processing technologies to achieve soft materials for in vitro tissue engineering and in vivo tissue regeneration.
- Design and Synthesis of Polymers: We create polymers with customized structures and bioactive functionalities to meet specific research and application needs.
- Fabrication of Soft Materials and Devices: Utilizing advanced techniques such as injection molding, electrospinning, and microfluidic wet-spinning, we develop innovative soft materials and devices.
- Biological Response Evaluation: We conduct comprehensive studies to assess the biological responses to our designed soft materials.
Kongchang Wei, Dr.
Scientific group leader
Keywords: polymer chemistry and physics; supramolecular biomaterials; tissue engnieering; microfluidics
Thomas Ramsauer, Dr.
Laboratory technician
Keywords: Polymer purification and analysis
Julia Hau, MSc
Laboratory technician
Keywords: Cell culture; Biomechanical testing
Tobias Hammer
PhD candidate, ETHZ, D-HEST
Project: Biomimetic membranes for in vitro skin models
Wuchao Wang
PhD candidate, ETHZ, D-HEST
Project: Microfluidic wet spinning of bio-based polymer fibers
Dardan Bajrami
(Guest) PhD candidate, ZHAW; ETHZ, D-HEST
Project: Photothermal-based skin model
Katharina Sribike
(Guest) PhD candidate, ETHZ, D-HEST
Project: Supramolecular hydrogels for in vitro tissue models
Dominique Grimm
PhD student, ETHZ, D-HEST
Project: Soft patch for "Swiss Cheese" ventricular septal defect closure
Anna-Lena Bauknecht
PhD student, ETHZ, D-HEST
Project: Soft patch for "Swiss Cheese" ventricular septal defect closure

Biointerfaces
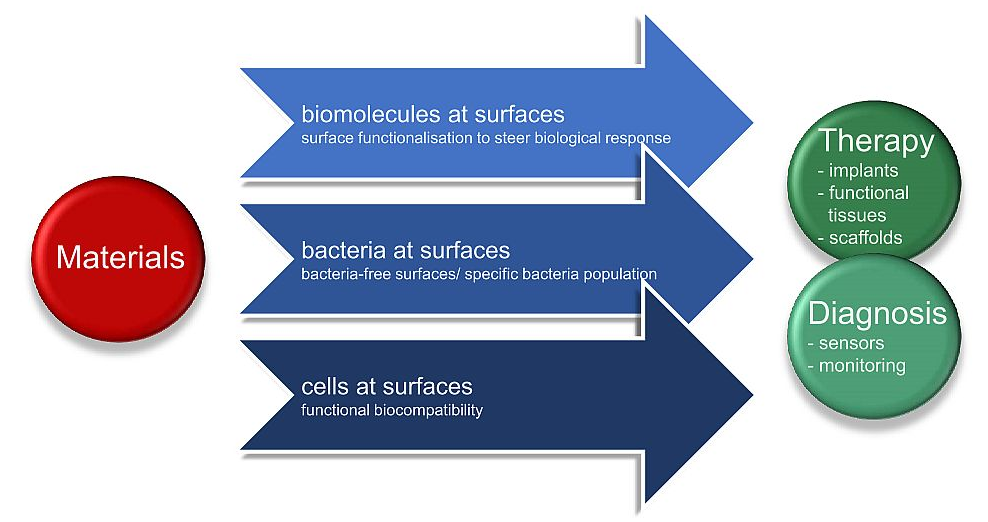
Our lab is active in research and development of novel materials-based healthcare solutions. We thrive to understand, characterise, and steer interactions of biomolecules, bacteria and human cells at materials surfaces. We study biointerfaces which encompass natural interfaces between biomolecules, their assemblies and water, between cells and extra cellular matrix, between populations of bacteria and human cells and their surroundings and those between the biological environment and materials for medical applications.
Designed biointerfaces are vital elements for the functionality of bio-related processes and devices. We carry out research on biointerfaces and through this develop materials for biosensors, diagnostics, biomimetic materials, (stem) cell technology, drug-delivery systems, materials for regenerative medicine, and biomaterials for medical implants and functional tissue engineering.
We steer the properties and nature of the biointerface by combining materials and biological science for healthcare solutions. With this we provide key contributions to novel antifouling, antibacterial and functional biomaterials for therapeutic and diagnostic use, such as implants, wound healing materials or sensors.