Materials science meets medicine
Why implants fail
What happens to titanium implants once they are inside the human body? Why are they sometimes rejected or even break? Empa researcher Martina Cihova is exploring these questions at the interface between implants and human tissues and cells, where materials science meets medicine. She was recently awarded an Ambizione Grant from the Swiss National Science Foundation (SNSF) to support her research.

Thanks to advances in medicine, people today live longer than ever. Understandably, we also want to remain healthy and mobile well into old age. Implants and prosthetics can replace worn joints and teeth, relieve pain and greatly improve our quality of life. Modern medical implants are small marvels of biomaterials science and bioengineering. Yet, despite their sophistication, implants do occasionally fail, which can have serious consequences for patients.
Why do these failures occur – and why have they been occurring more frequently in recent years? Empa researcher Martina Cihova from the Joining Technology and Corrosion laboratory aims to find out. To do so, she is taking a close look at the behavior of implants inside the body. The scientist has received a four-year Ambizione Grant from the Swiss National Science Foundation for her research project.
Many commonly used implants – including artificial joints, dental implants and pacemakers – are made of titanium. This transition metal is lightweight and strong, highly durable inside the body, and particularly good in allowing bone tissue to attach to it. These favorable properties are due to a thin oxide layer that forms naturally on the surface of titanium when exposed to air. Ultimately, it is not the titanium itself, but this protective surface layer that comes into contact with the body. “As this native passive layer is less than ten nanometers thick, it often receives too little attention in medical technology and research,” says Martina Cihova.
In addition, some manufacturers modify the oxide layer, for example, by thickening it to color-code implant types and sizes. Others roughen the surface of the implants to encourage bone integration – or laser-engrave the serial number to ensure traceability. Thanks to laser-based processes, even 3D printing of patient-specific implants is now possible. These are all useful applications, but: “Any treatment can change the titanium oxides on the implant surface,” says Cihova, “and there has been far too little research into how these changes affect the interaction of the implant with the body and its corrosion resistance.”
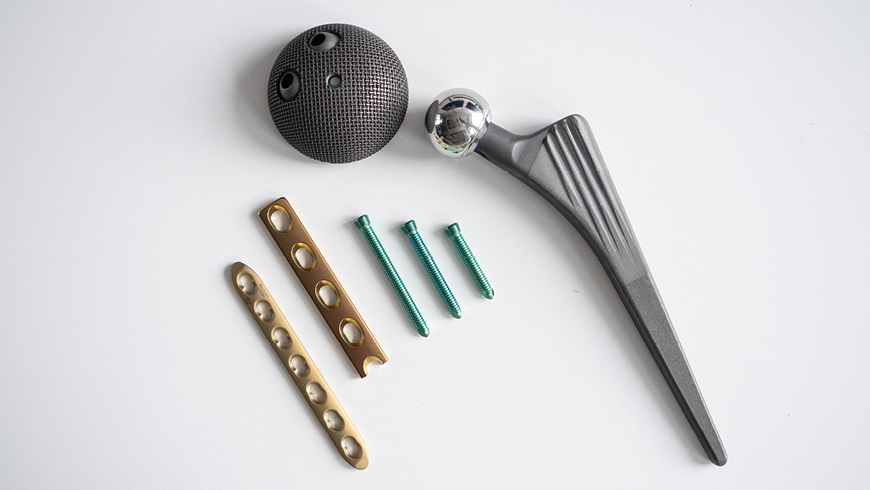
Research on the edge
With her project, the Empa researcher aims to close this knowledge gap. Her fascination with materials science began during her studies in bioengineering, which inspired her to shift focus and pursue a PhD in metallurgy to dive deeper into the world of materials. Today, she combines both fields of expertise, focusing on where metal, or metal oxides, and biology meet: the interface between implants and the human body.
“Such biointerfaces are incredibly complex, but also extremely fascinating,” says the young researcher. “When we think of corrosion, we usually think of salty seawater, humid air, maybe a rusty bicycle – but not the human body.” Yet our body can be a surprisingly aggressive environment to materials, especially when immune reactions take place. Immune cells release various substances that can, for instance, lower the pH value and attack the implant. So, what effect does the body's environment have on materials that we consider stable? This is the question at heart of biocorrosion research.
These processes are very complex on both the (electro)chemical and the biological level. In addition, not all titanium oxide is the same. Although its chemical composition, TiO2, remains the same, titanium oxide can exist in three different crystalline forms or in an amorphous, “undefined” structural state. Each of these forms has different electronic and electrochemical properties, which may also affect how the material interacts with the body.
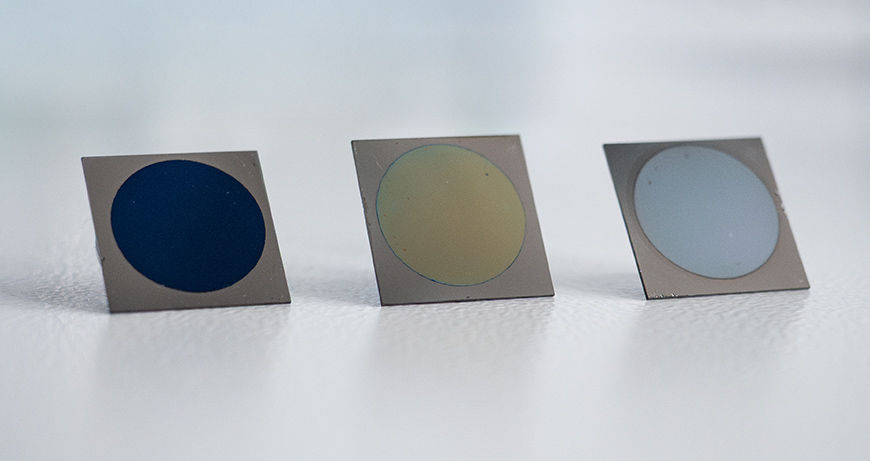
A stepwise approach to complexity
The surface treatment of implants can alter the crystalline structure of these oxides, either across the entire implant or only in selected areas. To understand how this local heterogeneity affects the already complex biointerface, Cihova and her team are taking a systematic step-by-step approach. In collaboration with experts for laser processing of metals at Empa in Thun, they create sample substrates with differently structured titanium oxide layers that vary systematically in their heterogeneity. These substrates are then exposed to increasingly complex body fluids to investigate the fundamental relationships between oxide structure, properties and reactivity.
“We start with simulated physiological fluids that contain only water and ions,” explains Cihova. The next step involves adding proteins such as fibrinogen, which is involved in immune responses and wound healing. Finally, the researchers plan to investigate how the biointerface behaves in contact with living macrophage cells – the body's police force. For this, they are collaborating with Empa researchers in St. Gallen. "I am very pleased that we were able to get colleagues from all three Empa sites on board for this project," says Cihova. "The complex questions we are addressing are inherently interdisciplinary, and tackling them requires expertise from across multiple fields."
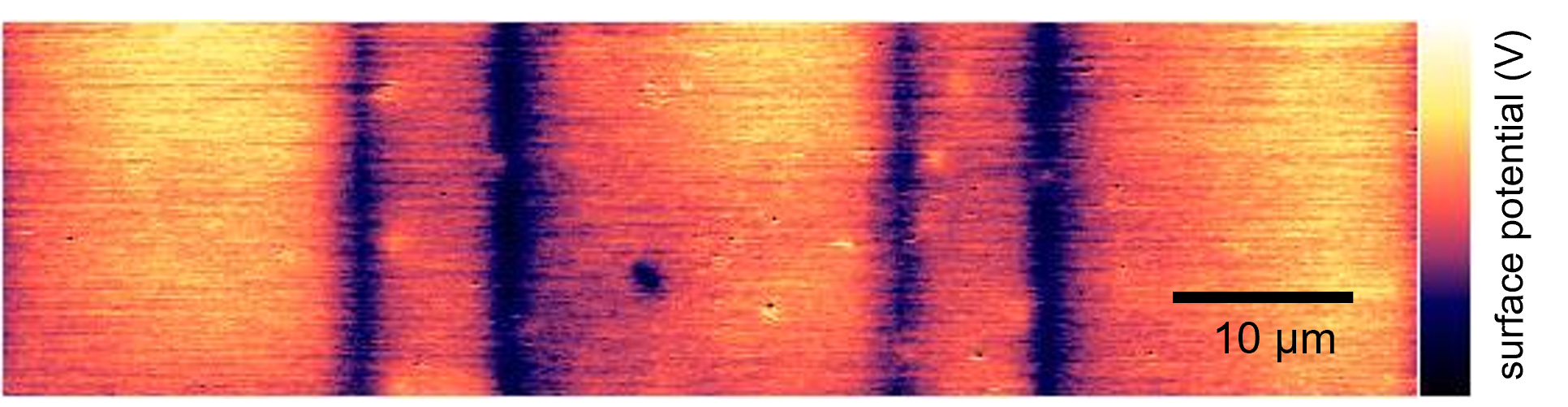
At each stage of the study, the interfaces are thoroughly investigated using electrochemical methods paired with high-resolution electron and atomic force microscopy. "Seeing is understanding – even if that means zooming in to length scales much smaller than a human cell,” says Cihova. "That is where crucial details can often be uncovered."
The Empa researcher hopes that the findings from the next few years will lead to safer, more reliable implants. And also "that we learn more about how to effectively harness the remarkable variety of oxide properties for specific biomedical applications." Following her Ambizione project in 2028, she plans to expand the new methods to other medical materials. Cihova is convinced that this field of research will become even more important in the future: "The behavior of metal oxides at biointerfaces is also key to their performance in the emerging fields of nanomedicine and implantable sensor technology."
Dr. Martina Cihova
Joining Technologies & Corrosion
Phone +41 58 765 43 88
Surfaces and Interfaces
Totally superficial? You bet! What might be a bit of a character flaw in a person is actually a good thing in materials science. Because, chemically speaking, it's what happens on the surface that counts. A sound understanding of surfaces and interfaces enables everything from better electronics to more robust bridges. Whether it's biological compatibility in implants, antibacterial coatings in hospitals, or catalytic processes in the production of synthetic fuels – surfaces are crucial to all of these.
Read the latest EmpaQuarterly online or download the PDF version.
-
Share






