Platform chemicals from CO2
Defects welcome
Is it possible to convert CO2 back to fuels or other useful chemicals? Absolutely – but not in a very targeted way just yet. Empa researcher Alessandro Senocrate is looking at defects in materials that will help us achieve this goal.

Can we undo the burning of oil, gas and coal? With a renewable source of electricity, some water and a suitable catalyst, the excess CO2 in the atmosphere could become a resource, for example for the production of synthetic fuels, so-called synfuels.
This promising idea is drawing a lot of research, including at Empa, because the conversion is challenging to implement. For instance, a reaction using a copper catalyst – the most commonly studied catalyst for the conversion of carbon dioxide – yields around 15 to 20 different types of molecules, from carbon monoxide and methane to propanol and acetic acid. "Some of these compounds are liquid at room temperature, others are gaseous," says Empa researcher Alessandro Senocrate. "It is extremely difficult to separate all these products from each other."
Senocrate, who works in Empa's Materials for Energy Conversion laboratory led by Corsin Battaglia, intends to investigate a possible solution to this problem over the next four years. The project is funded by a Swiss National Science Foundation (SNSF) Ambizione grant and embedded thematically into the National Center of Competence in Research (NCCR) Catalysis. The goal of the project is to develop novel catalysts for CO2 conversion. For this, Senocrate is focusing not on the material itself, but on so-called defects. Defects in a crystalline material occur, e.g., when an atom is missing in its crystal lattice or is replaced by a different atom. These defects introduce different properties in their host materials, and thus can serve as unique active sites, where catalysis takes place.
Where batteries aren't an option
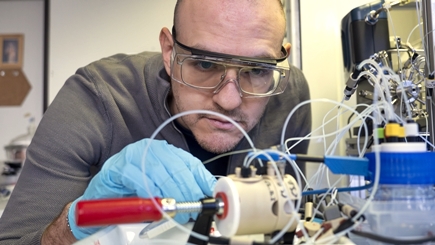
First, the researcher plans to investigate which defects lead to which reaction products. "Ideally, we can use this knowledge to design catalysts that produce specific molecules during the CO2 conversion instead of a mixture," he explains. Some of the potential target molecules are of particular interest to industry. These include carbon monoxide and ethylene. These molecules are so-called platform chemicals: They are the starting materials for numerous chemical processes, including the production of most plastics. "We already have a complete value chain for such platform chemicals," says Senocrate. "However, today they are almost exclusively produced from fossil sources." Alternative, greener sources for carbon-based chemicals – whether from CO2 conversion or from biomass – are thus in high demand.
In addition to plastics, platform chemicals can also be used to make fuels. Other research projects at Empa focus on the production of synfuels. "Cars can be electrified very well," says Alessandro Senocrate. "But it's a different story for aircraft and for many energy-intensive industrial processes."
The advantage of liquid fuels such as kerosene is their very high energy density, which can exceed that of batteries by a factor of up to 100. Fuels produced with renewable energy are therefore also an especially attractive option for seasonal energy storage. The infrastructure for the transportation and storage of synfuels is already in place, as they are almost identical to fossil fuels in terms of their composition. The only thing missing is the ability to produce them on a large scale. Senocrate is optimistic: "I've only been working in this field for a few years, and yet I have already seen incredible progress," says the scientist. "Of course, it will also require major political and societal change. But from a materials science perspective, the progress is rapid."
Perfecting the technology
Before Senocrate can contribute to this progress with his Ambizione project, he still has a few challenges to overcome. One of the biggest: introducing enough defects into the target material to achieve a measurable catalytic effect. This is because the researcher deliberately uses an inert starting material that has no influence on the electrochemical reaction without the defects. "This allows me to determine very precisely what effect the respective defects have," he explains.
Once the defects have been characterized, they can also be inserted into existing catalytic materials. "Ideally, at the end of the project, we will be able to improve an existing system for CO2 conversion in a targeted manner," says Senocrate. Such systems are already in use in the Materials for Energy Conversion lab, where researchers are developing different catalysts and electrode materials.
The demands placed on these materials are high: "For industrial use, the catalyst must be selective, active and stable," explains Senocrate. Selectivity means that it only produces a single chemical – or at least a small number of chemicals that can be easily separated. High activity is required to produce a maximum amount of chemicals or fuels with minimal energy. And, of course, a market-ready catalyst should maintain high selectivity and activity over thousands of operating hours, i.e. be stable. "We still need to get much better at all three properties," says the researcher. "But we are making progress."
Dr. Alessandro Senocrate
Materials for Energy Conversion Laboratory
Phone +41 58 765 41 48
Dr. Corsin Battaglia
Materials for Energy Conversion Laboratory
Phone +41 58 765 41 31

Empa Quarterly#82 Mining the Atmosphere
To limit climate change, we need to compensate not only for future emissions, but also for historical ones. One solution would be the "atmospheric vacuum cleaner": we remove the excess CO2 from the atmosphere. But what do we do with it? Instead of extracting the carbon for polymers, medicines, fibers, fuels and the like from crude oil, we use atmospheric CO2. This is the simple – yet extremely challenging in technical terms – idea behind Empa's new research initiative, Mining the Atmosphere.
Read the EmpaQuarterly online or download the pdf-version.

Empa Quarterly#83 Perovskites: Versatile cristals
Over 180 years ago, a curious crystal was discovered in the Ural Mountains. Today, it has given rise to an entire class of materials that is of great interest to researchers: perovskites. What all perovskites have in common is their crystal structure, which gives them unusual properties. By changing the exact composition of the perovskite, scientists can control these properties. Empa researchers are using this promising material to develop solar cells, detectors and quantum dots.
Read the EmpaQuarterly online or download the pdf-version.
-
Share

|
Embracing change Nathalie Casas took over the lead of Empa's Energy, Mobility and Environment research department. She believes that humanity can save the climate from collapse – but that it will take more than "just" technology. |
|
Packaged in beer Empa researchers have extracted nanocellulose from a waste product of beer brewing and processed it into an aerogel. The high-quality biodegradable material could be used in food packaging. |