One step ahead towards the artificial heart
Muscles from the spray can
An artificial heart would be an absolute lifesaver for people with cardiac failure. However, to recreate the complex organ in the laboratory, one would first need to work out how to grow multi-layered, living tissues. Researchers at Empa have now come one step closer to this goal: by means of a spraying process, they have created functioning muscle fibers.
Anyone who requires a transplant because of cardiac failure must hope for a suitable donor organ. An artificial heart that does not trigger any rejection reactions in the body after implantation would be an elegant alternative. The Zurich Heart project of the research alliance University Medicine Zurich, of which Empa is a partner, is currently developing such an artificial heart. To ensure that the laboratory-made pump is tolerated by the body, the aim is to envelop and coat it in human tissue, much like a cloak of invisibility. Until now, the culturing of multi-layered functional tissues has been a major challenge in the up-and-coming area of "Tissue Engineering". Empa researchers have now succeeded in letting cells develop into muscle fibers in a three-dimensional synthetic polymer scaffold.
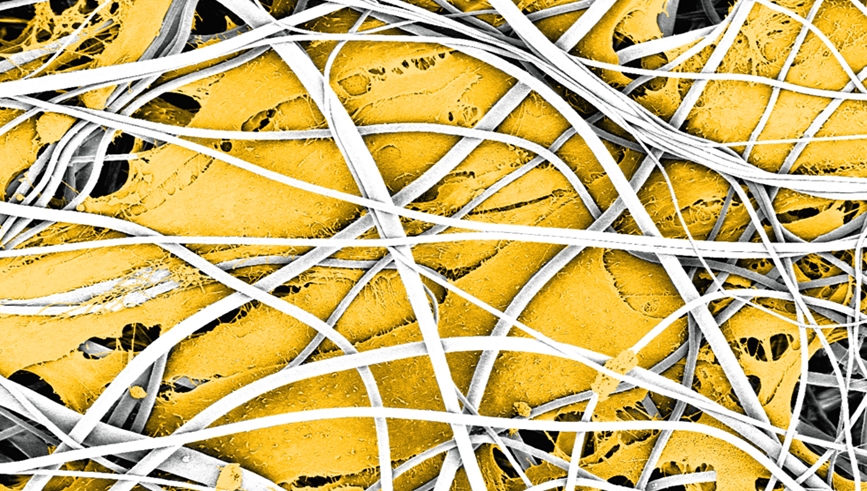
"The human heart is naturally composed of several layers of different tissues," explains Lukas Weidenbacher of Empa's laboratory for Biomimetic Membranes and Textiles in St. Gallen. Muscle fibers in the lining play a decisive role in the structure, for they are responsible for the stability and flexibility of the constantly beating heart. Culturing muscle fibers that grow in multiple layers is challenging, however, because the cells must first be embedded in a three-dimensional scaffold. "To be sure, it is possible to create three-dimensional polymer structures that closely resemble human tissue, by means of so-called electrospinning for example," says Weidenbacher. During this process, gossamer-like threads of liquid polymer are interlaced in the manner of natural tissue. But the harmful solvents that are required for this process are poison for the sensitive cells.
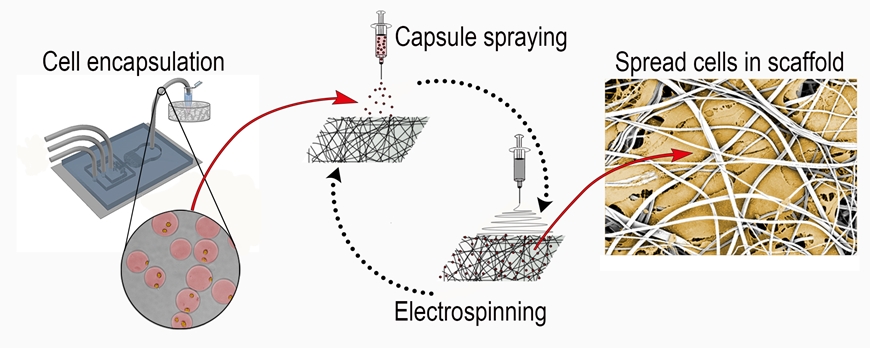
Slobbery protection
The researchers at Empa have therefore packaged the valuable cells in protective capsules. Gelatin sheaths contain one to two cells each. This protects the cells from the solvents. A special spraying process, called electrospraying, makes it possible to inject the capsules into the pores of the spun scaffold. "Cells that are protected in this way survive the spraying very well," explains the materials scientist. And once the cells have settled at the desired location, the gelatinous capsule dissolves within minutes.
After electrospraying, the gelatinous capsule (light green) dissolves within
minutes, while the cells (orange) stay active and alive. (via GIPHY)
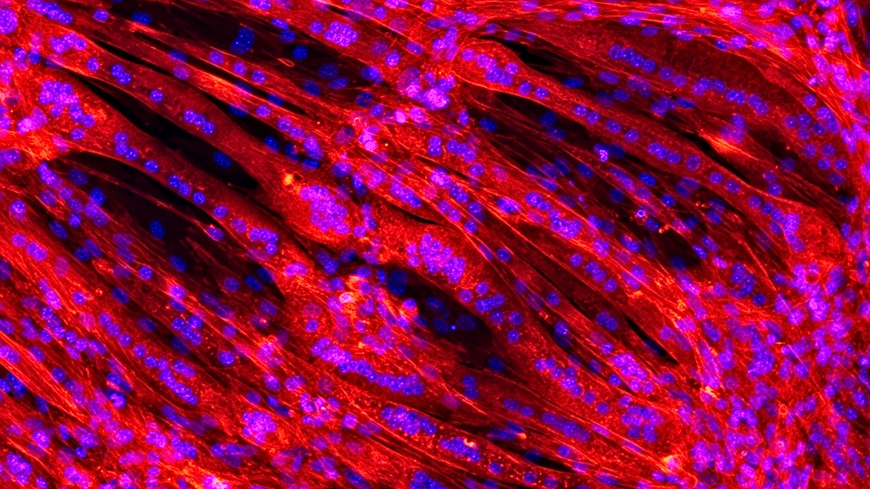
Scanning electron microscope images show that the cells feel at home in their synthetic polymer nest: As soon as the capsules have dissolved, the immature precursor cells begin to join up and to mature to form elongated muscle fibers. The aim is to end up with a structure that resembles natural muscle tissue as closely as possible. "As the artificial heart is constantly perfused by the blood circulation, it is important that the surfaces are of a quality that prevents coagulation," says Weidenbacher.
Invisible to the immune system
The researchers have used the immature muscle cells of a mouse cell line for their series of experiments. These precursor cells differentiated in the scaffold and produced proteins that normally occur in muscle. However, in the future the aim is to clad the implantable artificial heart with cells that derive from the patients themselves. In this way, a personal heart could be grown for the patient that remains "invisible" to the body's immune system.
L Weidenbacher, A Abrishamkar, M Rottmar, AG Guex, K Maniura-Weber, AJ deMello, SJ Ferguson, RM Rossi, G Fortunato; Electrospraying of microfluidic encapsulated cells for the fabrication of cell-laden electrospun hybrid tissue constructs; Acta Biomaterialia (2017); doi: 10.1016/j.actbio.2017.10.012
Heart failure is an increasing and serious problem in Western populations. The project aims at a fully implantable artificial heart: the "Zurich Heart“. Nearly 20 research groups of ETH Zurich, the University of Zurich, the associated hospitals in Zurich as well as the German Heart Center Berlin and Empa are committed to the ambitious vision.
-
Share
