Redox flow batteries
Renewable energy is expanding rapidly, with over 90% of new global capacity in 2024 coming from renewables, but its intermittency strains power grids, making large-scale energy storage essential to balance supply and demand. Redox flow batteries offer a safe, flexible alternative to lithium-ion batteries, storing energy in water-based electrolytes for durations from seconds to days. Their independently scalable power and capacity make them ideal for deployments in buildings, urban areas, or near critical infrastructure. State-of-the-art flow batteries use vanadium, a costly critical raw material with a concentrated supply chain, limiting their potential to scale.
Our research focuses on advancing redox flow batteries beyond the limits of current vanadium batteries. We develop redox targeting strategies that bypass solubility constraints by incorporating solid capacity boosters, enabling more compact energy storage (a-b). In parallel, we design metal-organic chelates for high-voltage, high-power flow battery chemistries that are cost-effective and free from critical raw materials. Finally, we functionalize electrodes to enhance reaction kinetics and mass transport, boosting both power output and energy efficiency (c). Together, these approaches aim to deliver safe and cost-efficient flow battery technologies capable of supporting renewable integration from short-term balancing to multi-day storage.
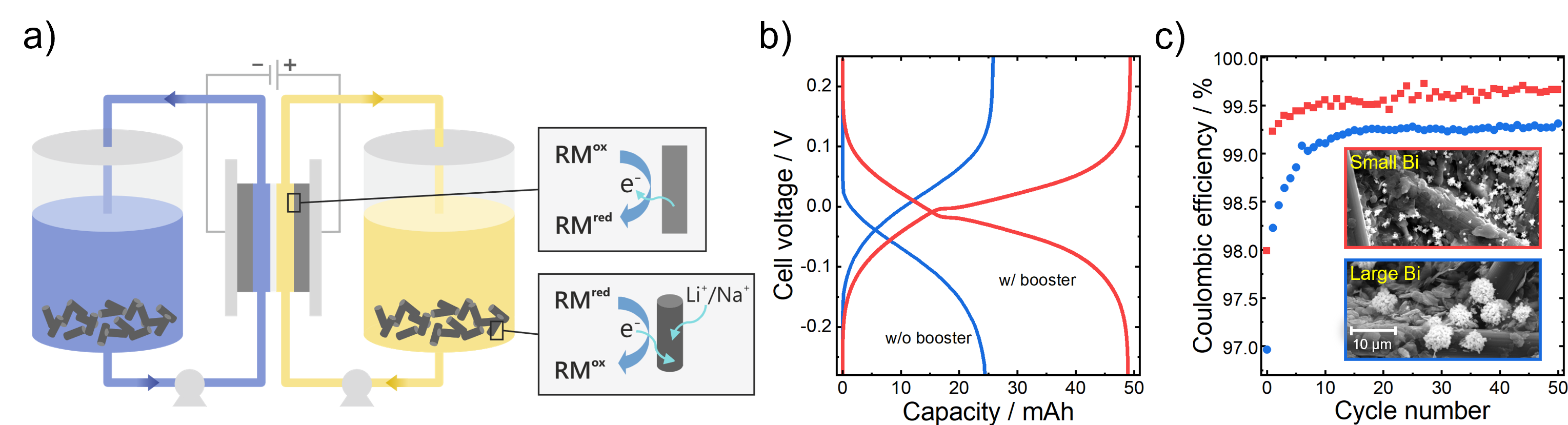
Research directions toward next-generation redox flow batteries: (a) Redox targeting enables energy densities beyond solubility limits by coupling dissolved redox mediators (RM) with solid capacity boosters. (b) Example voltage profiles of a symmetric cell showing increased accessible capacity when using a solid booster. (c) Electrode functionalization improves reaction kinetics and efficiency, as shown for electrodes decorated with different bismuth morphologies.
Selected publications
[1] J. R. Thurston, K. White, M. Kudisch, L. Iglesias Kitsu, J. Lorenzetti, F. Bernasconi, M. F. Toney, M. P. Marshak, D. Reber, Electronic structure distortions in chromium chelates impair redox kinetics in flow batteries, Batteries & Supercaps, 2025, e202500250, https://doi.org/10.1002/batt.202500250
[2] D. Reber, Tank costs are an overlooked factor in flow battery economics, Nature Energy 2025, 10, 23-27. https://doi.org/10.1038/s41560-024-01677-6
[3] T. Echeverria, F. Bernasconi, P. Ziemiański, D. Reber, Impact of thermal electrode activation on electrocata-lyst performance in KCrPDTA/K4Fe(CN)6 flow batteries. Batteries & Supercaps 2024, e202400696 https://doi.org/10.1002/batt.202400696
[4] Dr. Reber, S. R. Jarvis, M. P. Marshak, Beyond energy density: Flow battery design driven by safety and lo-cation. Energy Advances 2023, 2, 1357, https://doi.org/10.1039/D3YA00208J
[5] Dr. Reber, J. R. Thurston, M. Becker, M.P. Marshak, Stability of highly soluble ferrocyanides at neutral pH for energy dense flow batteries, Cell. Rep. Phys. Sci. 2022, 4, 101215, https://doi.org/10.1016/j.xcrp.2022.101215
Funding
Swiss National Science Foundation (https://data.snf.ch/grants/grant/209078)


Water-based flow batteries, this is a battery, https://www.empa.ch/web/s604/redox-flow-david-reber
-
Share
