Instruments & Equipments
Density and porosity analysis
The Accupyc 1340 uses an inert gas as the displacement medium in order to obtain the true, absolute, skeletal, and apparent volume and density. Gas pycnometry is non-destructive as it uses the gas displacement method to measure volume. Density calculations using the gas displacement method are much more accurate and reproducible than the traditional Archimedes water displacement method.
The GeoPyc 1360 utilizes a quasi-fluid displacement medium composed of non-hazardous microspheres having a high degree of flowability that do not wet the sample or fill its pores. This allows determining the envelope volume and density of monolithic samples as well as bulk volume and density of powdered materials.

Surface and pore size analysis
Nitrogen sorption is a method to determine nitrogen isotherms at the boiling point of liquid nitrogen (77.3 K). The shape and the height of the isotherm are characteristic for each porous sample and allow deriving quantitative information on the specific surface area and other structural parameters of the porous material under investigation. Prior to the measurement adsorbed gases and vapors such as water vapor are removed from the sample by heating it under vacuum. Starting from the evacuated state nitrogen is stepwise dosed into the sample holder containing the porous material under investigation. The sample is hereby kept at a constant temperature of 77.3 K by using a liquid nitrogen bath.
Micromeritics 3Flex Surface Characterization Analyzer is a fully automated, three-station instrument capable of high-performance physisorption (mesopore and micropore) analysis. The 3Flex is ideally suited for the characterization of MOFs, zeolites, activated carbons, adsorbents, and a wide variety of porous and non-porous materials.

Zeta potential and particle size analysis
The Zetasizer Nano ZS offers a high sensitivity, accuracy and resolution of the zeta potential. This is achieved by a combination of laser Doppler velocimetry and phase analysis light scattering (PALS) in Malvern's patented M3-PALS technique. Furthermore, non-invasive back scatter (NIBSR) technology allows to measure particles in the range of 0.6 nm to 6 μm.

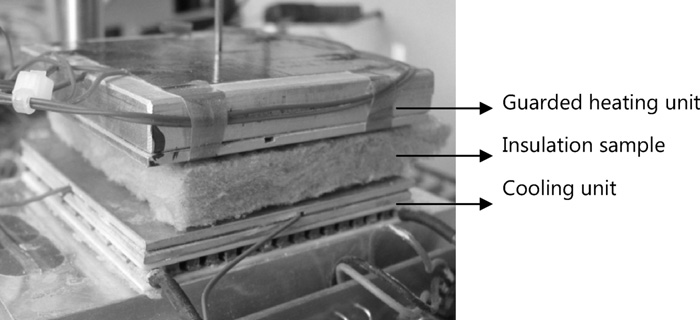
Thermal conductivity
A special hot plate device designed for small samples of low thermal conductivity materials is used for thermal conductivity measurements. The sample size used is 65 mm x 65 mm and 12-13mm in thickness. In order to be consistent with measurements according to the European Standards calibration measurements were carried out using conventional expanded polystyrene samples measured once in the standard test equipment and then cut into smaller pieces to be measured in a second run in the smaller apparatus.
Supercritical Drying
Aerogel technology provides high added-value lightweight materials with outstanding surface area and open porosity, susceptible of being used in a broad range of applications. However, one major problem for the preparation of the aerogels is to eliminate the liquid solvent from the gel avoiding the collapse of the already existing structure with the subsequent shrinkage and cracking of the dried gel. Upon solvent removal, the surface tension of the liquid contained in the gel pores will infer a capillary pressure gradient in the pore walls achieving pressures up to 100-200 MPa, able to collapse the pores. Hence, traditional drying procedures, e.g., air drying, are not able to preserve the gel structure.
Aerogels are usually obtained from wet gels by using the supercritical drying technology, able to avoid the pore collapse phenomenon in order to keep intact the porous texture of the wet material. Supecritical drying transforms the liquid contained in the gel into a supercritical fluid. The inherent null surface tension of supercritical fluids avoids the pore collapse phenomenon in the gel structure during solvent elimination.



